
Question Number 10282 by Tawakalitu ayo mi last updated on 02/Feb/17
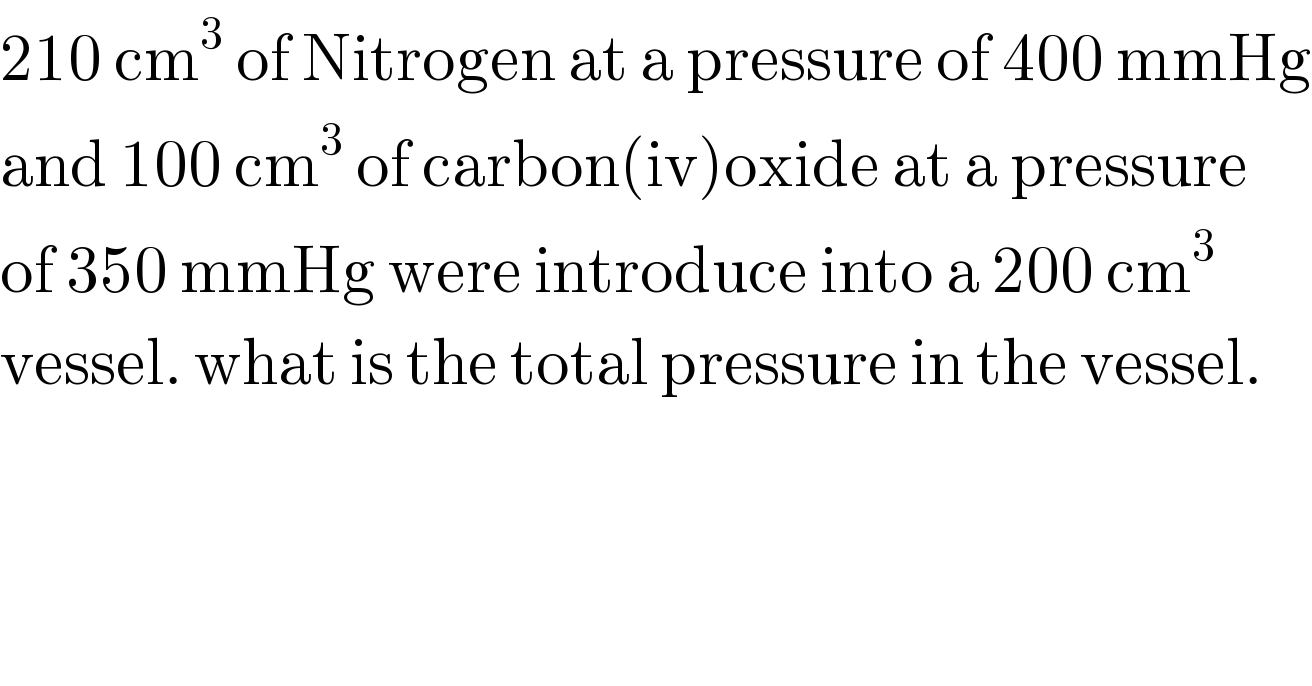
$$\mathrm{210}\:\mathrm{cm}^{\mathrm{3}} \:\mathrm{of}\:\mathrm{Nitrogen}\:\mathrm{at}\:\mathrm{a}\:\mathrm{pressure}\:\mathrm{of}\:\mathrm{400}\:\mathrm{mmHg} \\ $$$$\mathrm{and}\:\mathrm{100}\:\mathrm{cm}^{\mathrm{3}} \:\mathrm{of}\:\mathrm{carbon}\left(\mathrm{iv}\right)\mathrm{oxide}\:\mathrm{at}\:\mathrm{a}\:\mathrm{pressure}\: \\ $$$$\mathrm{of}\:\mathrm{350}\:\mathrm{mmHg}\:\mathrm{were}\:\mathrm{introduce}\:\mathrm{into}\:\mathrm{a}\:\mathrm{200}\:\mathrm{cm}^{\mathrm{3}} \\ $$$$\mathrm{vessel}.\:\mathrm{what}\:\mathrm{is}\:\mathrm{the}\:\mathrm{total}\:\mathrm{pressure}\:\mathrm{in}\:\mathrm{the}\:\mathrm{vessel}. \\ $$
Commented by Tawakalitu ayo mi last updated on 02/Feb/17
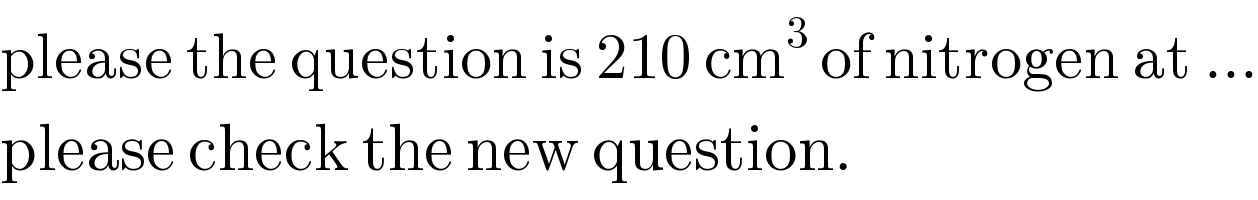
$$\mathrm{please}\:\mathrm{the}\:\mathrm{question}\:\mathrm{is}\:\mathrm{210}\:\mathrm{cm}^{\mathrm{3}} \:\mathrm{of}\:\mathrm{nitrogen}\:\mathrm{at}\:... \\ $$$$\mathrm{please}\:\mathrm{check}\:\mathrm{the}\:\mathrm{new}\:\mathrm{question}. \\ $$
Answered by ridwan balatif last updated on 02/Feb/17

$$\mathrm{The}\:\mathrm{total}\:\mathrm{pressure}\:\mathrm{is} \\ $$$$\mathrm{P}_{\mathrm{total}} =\frac{\mathrm{P}_{\mathrm{1}} \mathrm{V}_{\mathrm{1}} +\mathrm{P}_{\mathrm{2}} \mathrm{V}_{\mathrm{2}} }{\mathrm{V}_{\mathrm{1}} +\mathrm{V}_{\mathrm{2}} } \\ $$$$\:\:\:\:\:\:\:\:\:\:\:\:\:=\frac{\mathrm{400}×\mathrm{200}+\mathrm{350}×\mathrm{100}}{\mathrm{200}+\mathrm{100}} \\ $$$$\:\:\:\:\:\:\:\:\:\:\:\:\:=\frac{\mathrm{800}+\mathrm{350}}{\mathrm{3}}=\frac{\mathrm{1150}}{\mathrm{3}}\approx\mathrm{383}.\mathrm{3333mmHg} \\ $$
Commented by Tawakalitu ayo mi last updated on 02/Feb/17

$$\mathrm{wow}.\:\mathrm{God}\:\mathrm{bless}\:\mathrm{you}\:\mathrm{sir}. \\ $$
