
Question Number 203878 by Spillover last updated on 31/Jan/24
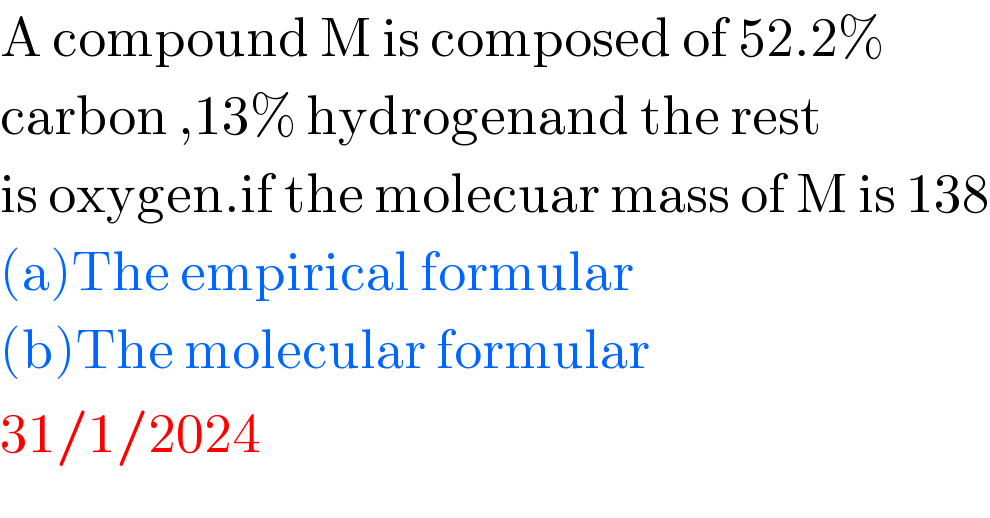
$$\mathrm{A}\:\mathrm{compound}\:\mathrm{M}\:\mathrm{is}\:\mathrm{composed}\:\mathrm{of}\:\mathrm{52}.\mathrm{2\%}\: \\ $$$$\mathrm{carbon}\:,\mathrm{13\%}\:\mathrm{hydrogenand}\:\mathrm{the}\:\mathrm{rest}\: \\ $$$$\mathrm{is}\:\mathrm{oxygen}.\mathrm{if}\:\mathrm{the}\:\mathrm{molecuar}\:\mathrm{mass}\:\mathrm{of}\:\mathrm{M}\:\mathrm{is}\:\mathrm{138} \\ $$$$\left(\mathrm{a}\right)\mathrm{The}\:\mathrm{empirical}\:\mathrm{formular} \\ $$$$\left(\mathrm{b}\right)\mathrm{The}\:\mathrm{molecular}\:\mathrm{formular} \\ $$$$\mathrm{31}/\mathrm{1}/\mathrm{2024} \\ $$
Answered by Calculusboy last updated on 31/Jan/24
![Solution: to get oxygen;100−(52.2+13)=34.8 empirical formula: c_((52.2)/(12)) h_((13)/1) o_((34.8)/(16)) 4.35 13 2.175 divide by smaller number 2 5.977 1 empirical formula : C_2 H_6 O molecular formula: [C_2 H_6 O]_n =138 [(12×2)+(1×6)+(16×1)]_n =138 [24+6+16]_n =138 46n=138 n=((138)/(46))=3 ∴molecular formula:[C_2 H_6 O]_3 =C_6 H_(18) O_3](Q203886.png)
$$\boldsymbol{{Solution}}:\:\boldsymbol{{to}}\:\boldsymbol{{get}}\:\boldsymbol{{oxygen}};\mathrm{100}−\left(\mathrm{52}.\mathrm{2}+\mathrm{13}\right)=\mathrm{34}.\mathrm{8} \\ $$$$\boldsymbol{{empirical}}\:\boldsymbol{{formula}}:\:\:\underset{\frac{\mathrm{52}.\mathrm{2}}{\mathrm{12}}} {\boldsymbol{{c}}}\:\:\:\underset{\frac{\mathrm{13}}{\mathrm{1}}} {\boldsymbol{{h}}}\:\:\:\underset{\frac{\mathrm{34}.\mathrm{8}}{\mathrm{16}}} {\boldsymbol{{o}}} \\ $$$$\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\mathrm{4}.\mathrm{35}\:\:\mathrm{13}\:\:\:\mathrm{2}.\mathrm{175}\:\:\:\: \\ $$$$\boldsymbol{{divide}}\:\boldsymbol{{by}}\:\boldsymbol{{smaller}}\:\boldsymbol{{number}} \\ $$$$\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\mathrm{2}\:\:\:\:\:\:\:\:\:\mathrm{5}.\mathrm{977}\:\:\:\:\mathrm{1} \\ $$$$\boldsymbol{{empirical}}\:\boldsymbol{{formula}}\::\:\:\boldsymbol{{C}}_{\mathrm{2}} \boldsymbol{{H}}_{\mathrm{6}} \boldsymbol{{O}} \\ $$$$\boldsymbol{{molecular}}\:\boldsymbol{{formula}}:\:\left[\boldsymbol{{C}}_{\mathrm{2}} \boldsymbol{{H}}_{\mathrm{6}} \boldsymbol{{O}}\right]_{\boldsymbol{{n}}} =\mathrm{138} \\ $$$$\left[\left(\mathrm{12}×\mathrm{2}\right)+\left(\mathrm{1}×\mathrm{6}\right)+\left(\mathrm{16}×\mathrm{1}\right)\right]_{\boldsymbol{{n}}} =\mathrm{138} \\ $$$$\left[\mathrm{24}+\mathrm{6}+\mathrm{16}\right]_{\boldsymbol{{n}}} =\mathrm{138} \\ $$$$\mathrm{46}\boldsymbol{{n}}=\mathrm{138} \\ $$$$\boldsymbol{{n}}=\frac{\mathrm{138}}{\mathrm{46}}=\mathrm{3} \\ $$$$\therefore\boldsymbol{{molecular}}\:\boldsymbol{{formula}}:\left[\boldsymbol{{C}}_{\mathrm{2}} \boldsymbol{{H}}_{\mathrm{6}} \boldsymbol{{O}}\right]_{\mathrm{3}} =\boldsymbol{{C}}_{\mathrm{6}} \boldsymbol{{H}}_{\mathrm{18}} \boldsymbol{{O}}_{\mathrm{3}} \\ $$$$ \\ $$$$ \\ $$
Commented by Spillover last updated on 11/Feb/24

$${correct} \\ $$
