
Atomic StructureQuestion and Answers: Page 1
Question Number 203877 Answers: 0 Comments: 0
Question Number 193437 Answers: 0 Comments: 0
Question Number 188808 Answers: 0 Comments: 2
Question Number 188775 Answers: 1 Comments: 0
Question Number 178019 Answers: 0 Comments: 0
Question Number 178018 Answers: 2 Comments: 0
Question Number 178013 Answers: 4 Comments: 0
Question Number 178011 Answers: 3 Comments: 0
Question Number 178009 Answers: 3 Comments: 2

Question Number 177606 Answers: 2 Comments: 0
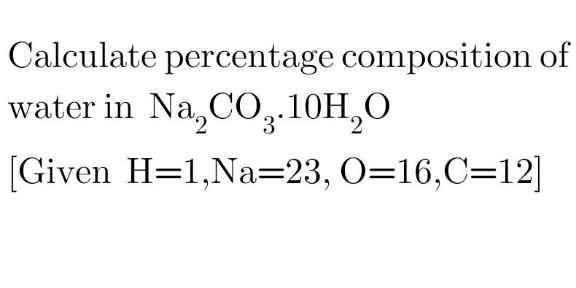
Question Number 177605 Answers: 2 Comments: 0

Question Number 166678 Answers: 1 Comments: 0
Question Number 159786 Answers: 0 Comments: 0
Question Number 159785 Answers: 0 Comments: 0
Question Number 135326 Answers: 0 Comments: 0

Question Number 125015 Answers: 2 Comments: 0

Question Number 121091 Answers: 0 Comments: 1

Question Number 90327 Answers: 0 Comments: 1
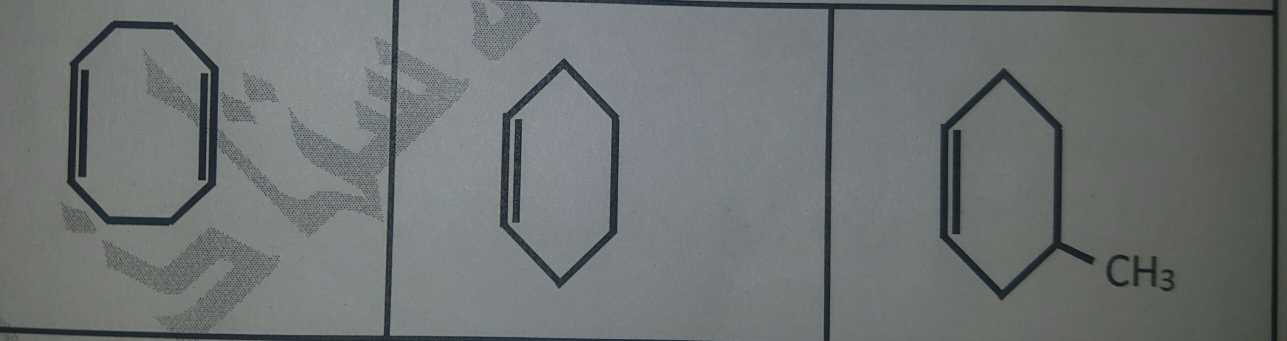
Question Number 77890 Answers: 0 Comments: 0

Question Number 75860 Answers: 1 Comments: 0
$${complete}\:{and}\:{balance}\: \\ $$$${S}+{HNO}_{\mathrm{3}} \rightarrow \\ $$$$ \\ $$
Question Number 64101 Answers: 0 Comments: 1
Question Number 53086 Answers: 0 Comments: 1
Question Number 52991 Answers: 1 Comments: 1

Question Number 50451 Answers: 2 Comments: 0
Question Number 32471 Answers: 0 Comments: 0

Question Number 32423 Answers: 0 Comments: 0

