
Heat and TheromdynamicsQuestion and Answers: Page 3
Question Number 42262 Answers: 1 Comments: 0
Question Number 42261 Answers: 1 Comments: 1
Question Number 42131 Answers: 1 Comments: 1
Question Number 42128 Answers: 0 Comments: 2
Question Number 42125 Answers: 0 Comments: 6
Question Number 41888 Answers: 0 Comments: 5
Question Number 40498 Answers: 1 Comments: 0
Question Number 39237 Answers: 0 Comments: 1
Question Number 39236 Answers: 0 Comments: 3
Question Number 39235 Answers: 0 Comments: 1
Question Number 34892 Answers: 0 Comments: 0

Question Number 34891 Answers: 1 Comments: 0
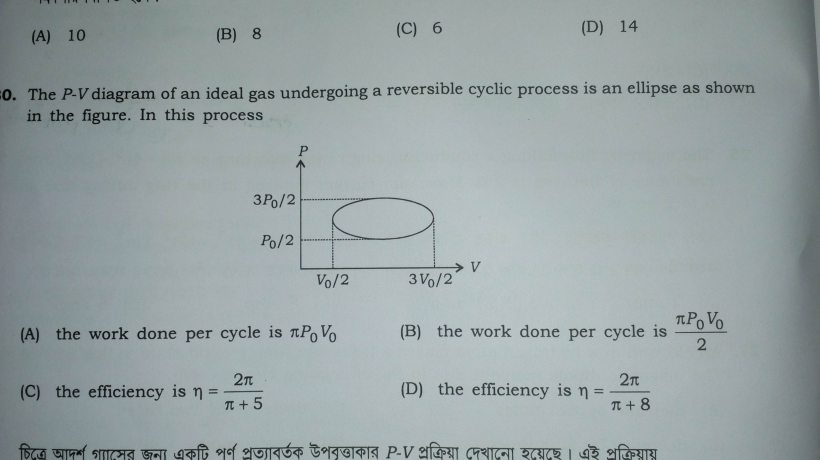
Question Number 32264 Answers: 0 Comments: 1
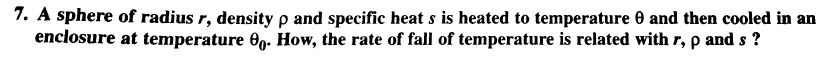
Question Number 31474 Answers: 1 Comments: 1
Question Number 31326 Answers: 1 Comments: 1

Question Number 31209 Answers: 1 Comments: 2
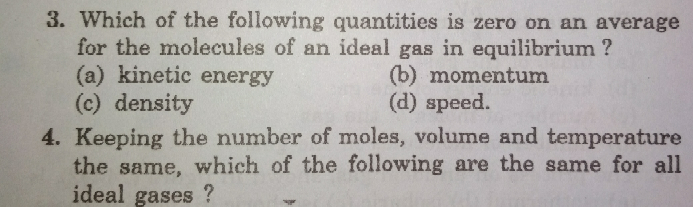
Question Number 31114 Answers: 1 Comments: 2

Question Number 27447 Answers: 1 Comments: 0
