
Ionic EquilibriumQuestion and Answers: Page 1
Question Number 141150 Answers: 0 Comments: 0
Question Number 114990 Answers: 0 Comments: 0
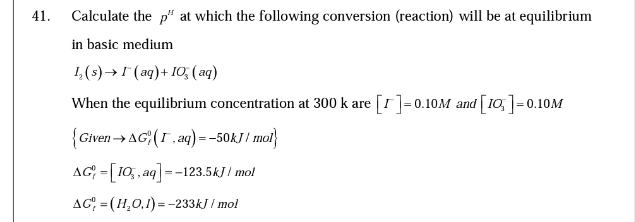
Question Number 36387 Answers: 0 Comments: 0
Question Number 31914 Answers: 0 Comments: 0

Question Number 31898 Answers: 0 Comments: 0

Question Number 31619 Answers: 1 Comments: 0

Question Number 31906 Answers: 1 Comments: 0

Question Number 31519 Answers: 1 Comments: 0
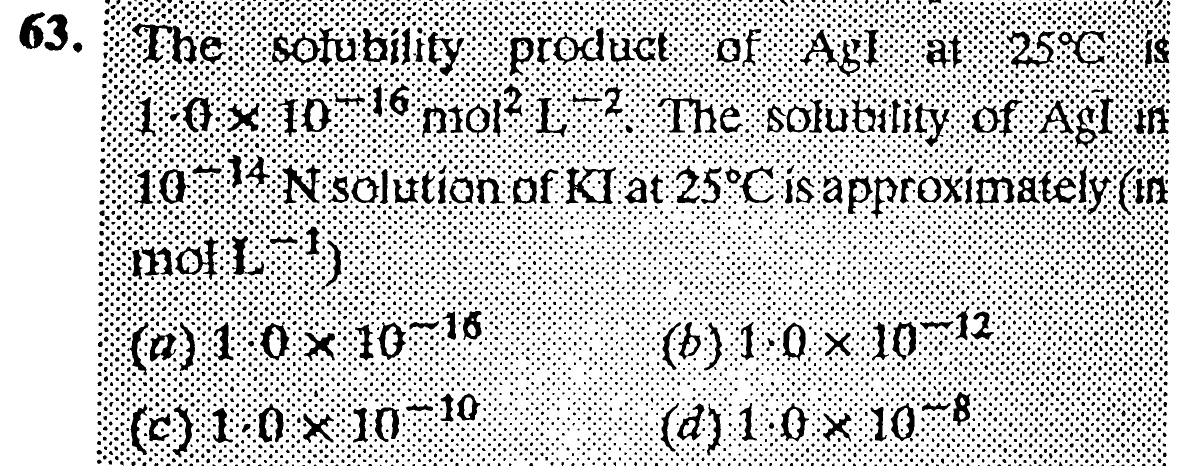
Question Number 31284 Answers: 0 Comments: 0
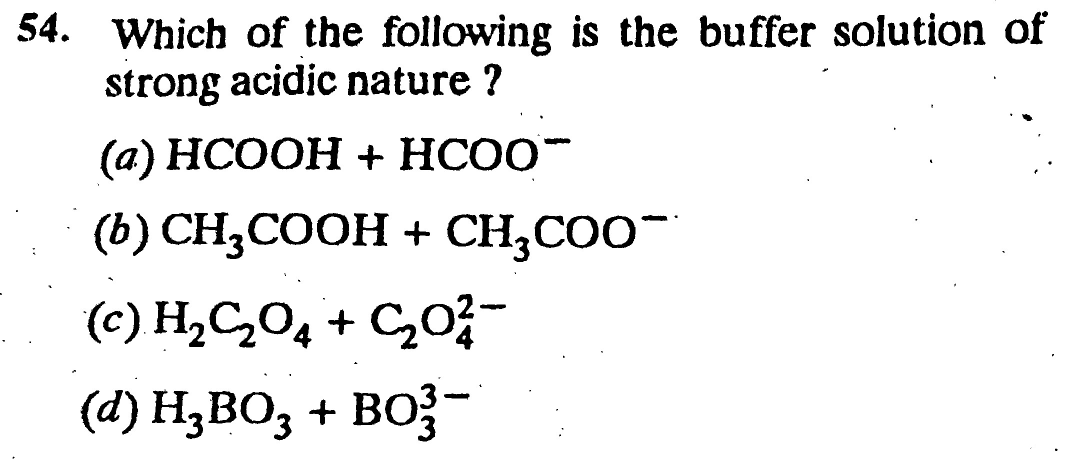
Question Number 30704 Answers: 0 Comments: 0

Question Number 30631 Answers: 0 Comments: 0

Question Number 30427 Answers: 0 Comments: 6
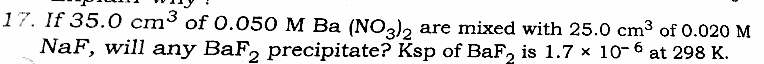
Question Number 29998 Answers: 0 Comments: 0

Question Number 29242 Answers: 0 Comments: 0

Question Number 27538 Answers: 0 Comments: 0

