
Organic ChemistryQuestion and Answers: Page 1
Question Number 176643 Answers: 0 Comments: 2
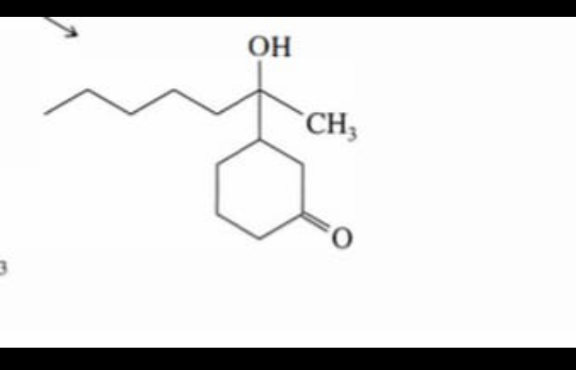
Question Number 176576 Answers: 0 Comments: 0
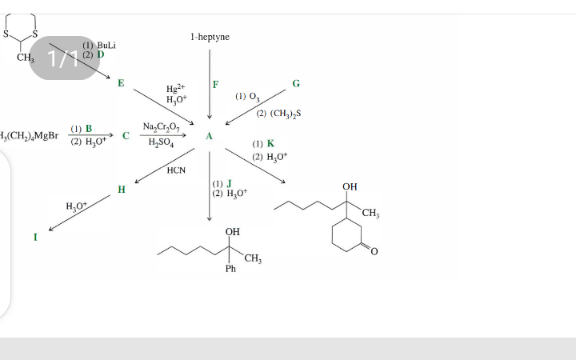
Question Number 174627 Answers: 0 Comments: 0

Question Number 174626 Answers: 0 Comments: 0

Question Number 174625 Answers: 0 Comments: 0
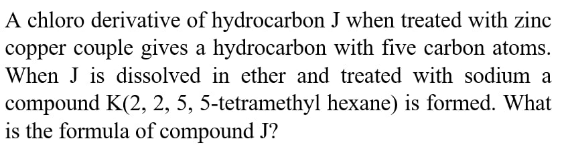
Question Number 174624 Answers: 0 Comments: 0

Question Number 174622 Answers: 0 Comments: 1

Question Number 174620 Answers: 0 Comments: 1
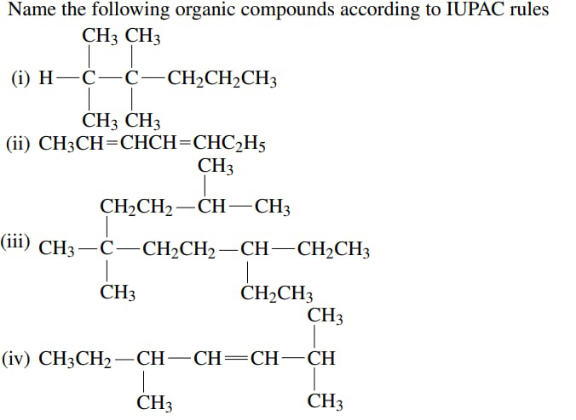
Question Number 164386 Answers: 0 Comments: 8
Question Number 135338 Answers: 1 Comments: 0
Question Number 134214 Answers: 0 Comments: 4

Question Number 128940 Answers: 1 Comments: 0
Question Number 128481 Answers: 0 Comments: 3
Question Number 104368 Answers: 1 Comments: 0
Question Number 104367 Answers: 1 Comments: 0
Question Number 104366 Answers: 0 Comments: 2
Question Number 57889 Answers: 0 Comments: 0
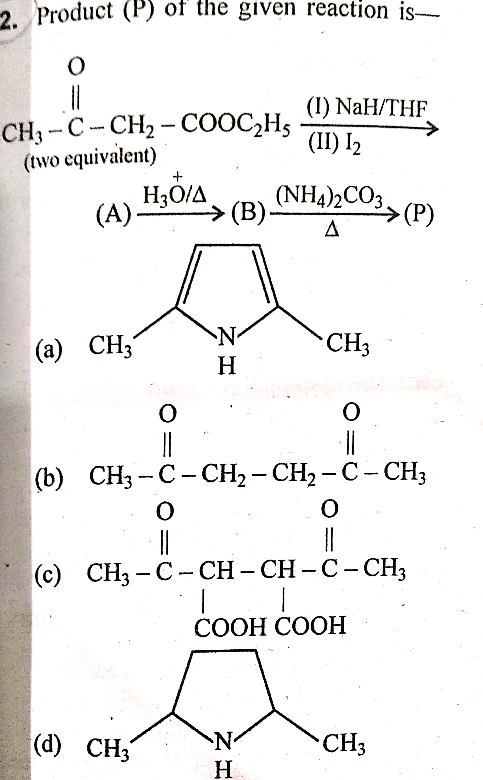
Question Number 57785 Answers: 1 Comments: 2
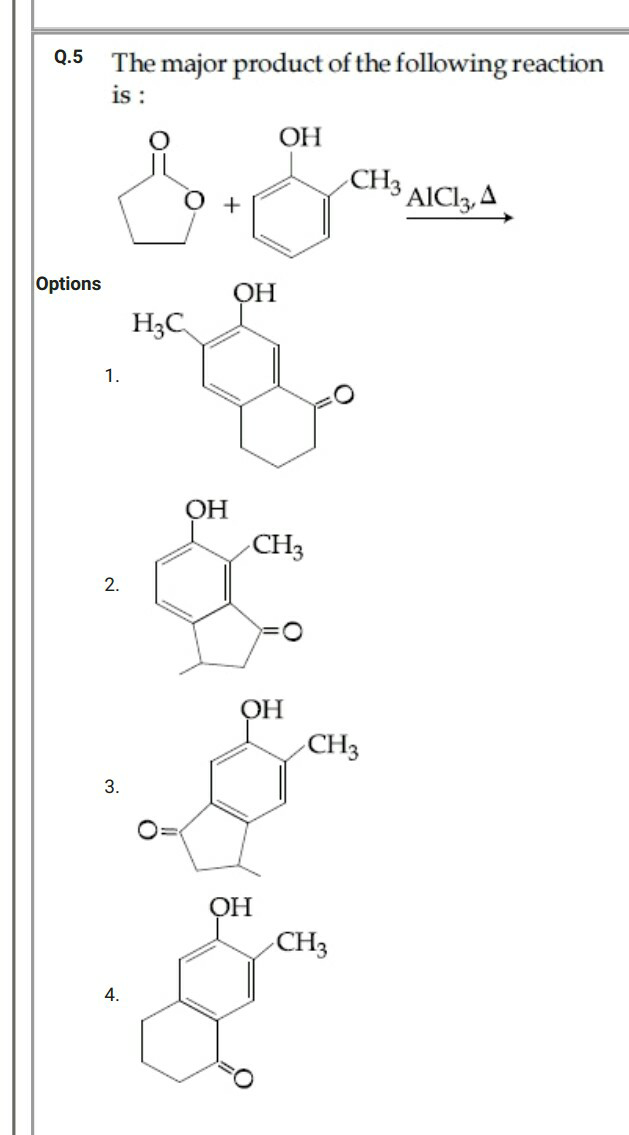
Question Number 55666 Answers: 0 Comments: 0
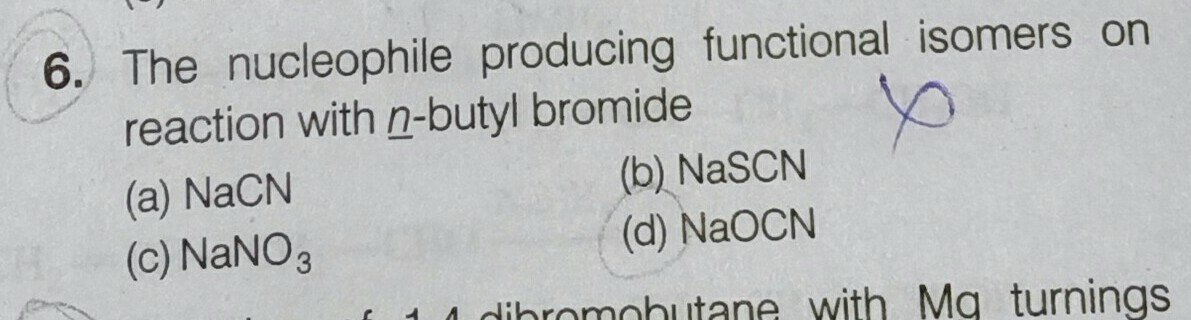
Question Number 55665 Answers: 0 Comments: 0

Question Number 54281 Answers: 0 Comments: 1
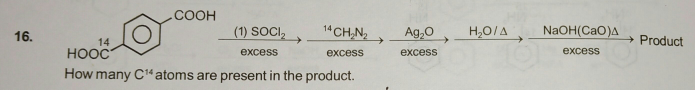
Question Number 53630 Answers: 1 Comments: 0
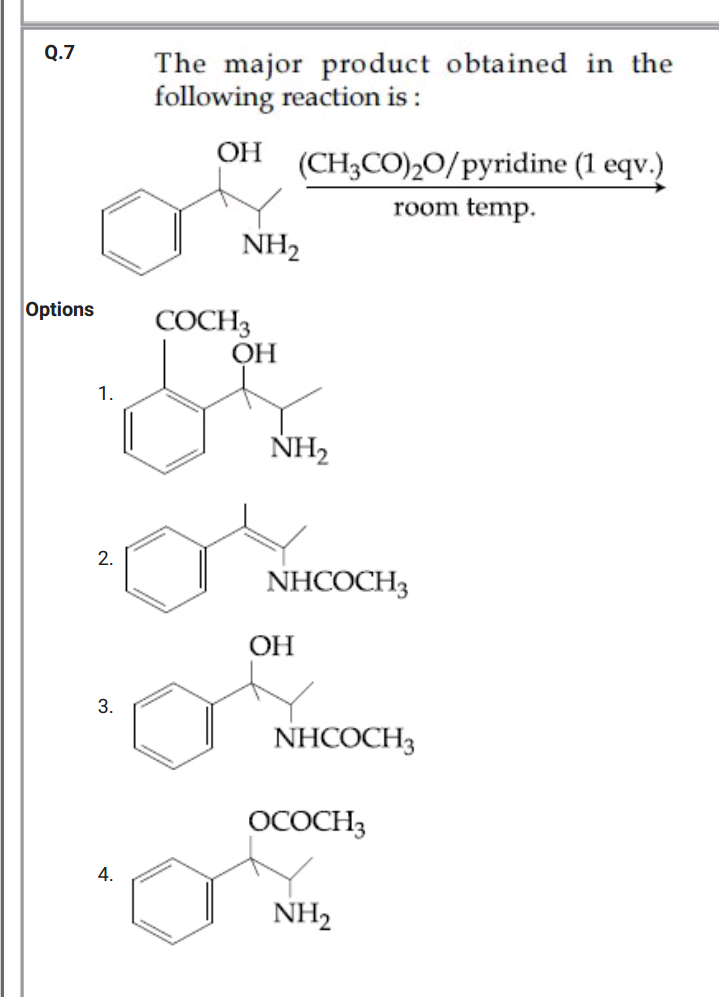
Question Number 51299 Answers: 0 Comments: 4
Question Number 51289 Answers: 1 Comments: 5
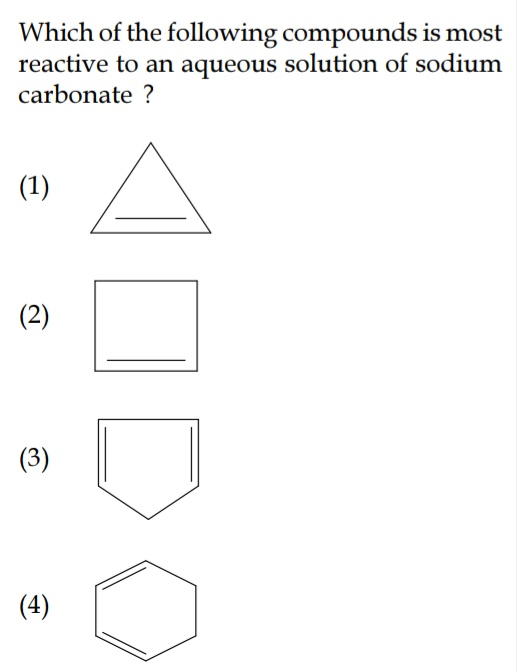
Question Number 38155 Answers: 0 Comments: 1
Question Number 37367 Answers: 0 Comments: 1

