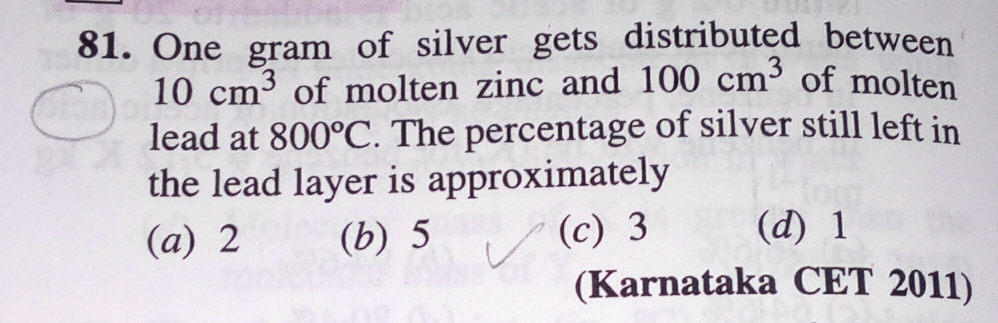SolutionsQuestion and Answers: Page 1
Question Number 189797 Answers: 0 Comments: 2

Question Number 178166 Answers: 1 Comments: 0
Question Number 178165 Answers: 2 Comments: 0
Question Number 152971 Answers: 0 Comments: 0

Question Number 136205 Answers: 0 Comments: 0

Question Number 112848 Answers: 0 Comments: 1
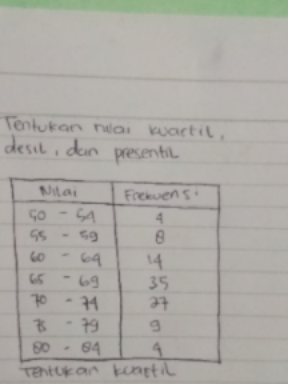
Question Number 69159 Answers: 0 Comments: 0
Question Number 64078 Answers: 0 Comments: 3

Question Number 64077 Answers: 0 Comments: 0

Question Number 62923 Answers: 2 Comments: 0

Question Number 62885 Answers: 0 Comments: 0

Question Number 52708 Answers: 0 Comments: 0

Question Number 52299 Answers: 0 Comments: 0

Question Number 52139 Answers: 0 Comments: 0

Question Number 41369 Answers: 0 Comments: 0