
Heat and TheromdynamicsQuestion and Answers: Page 2
Question Number 75166 Answers: 1 Comments: 0
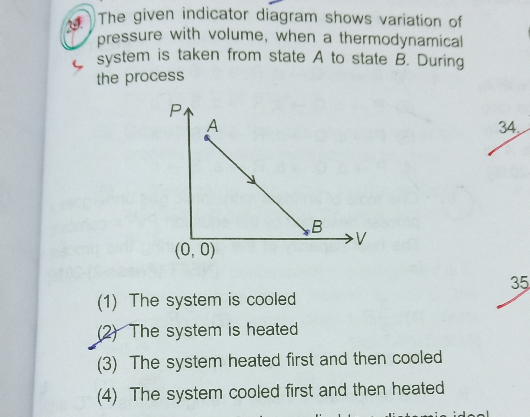
Question Number 74359 Answers: 0 Comments: 1
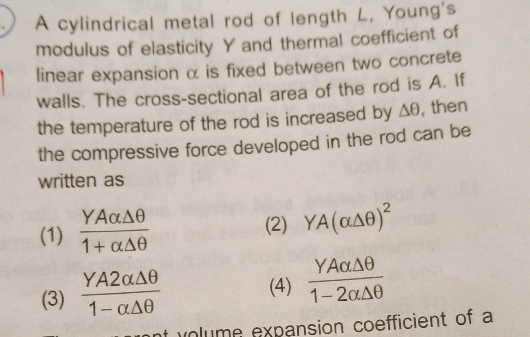
Question Number 74358 Answers: 0 Comments: 6
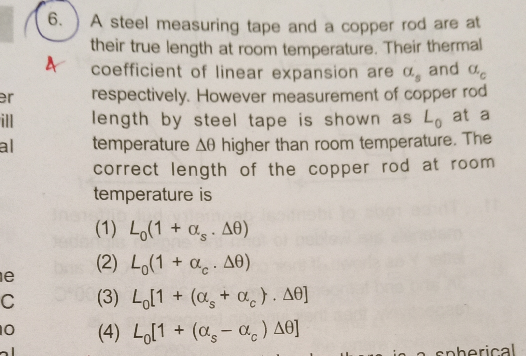
Question Number 71740 Answers: 0 Comments: 1
Question Number 71640 Answers: 0 Comments: 0

Question Number 70620 Answers: 0 Comments: 4

Question Number 59346 Answers: 0 Comments: 0
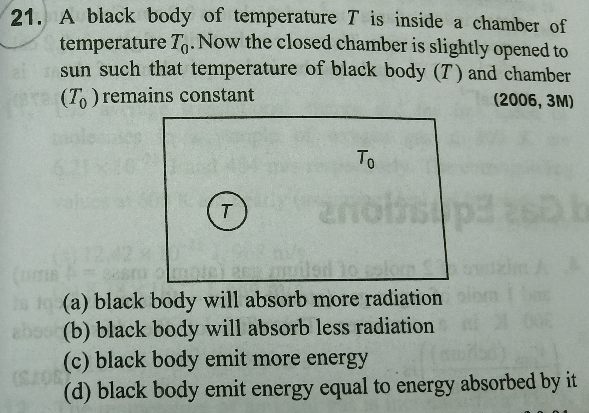
Question Number 58380 Answers: 0 Comments: 3
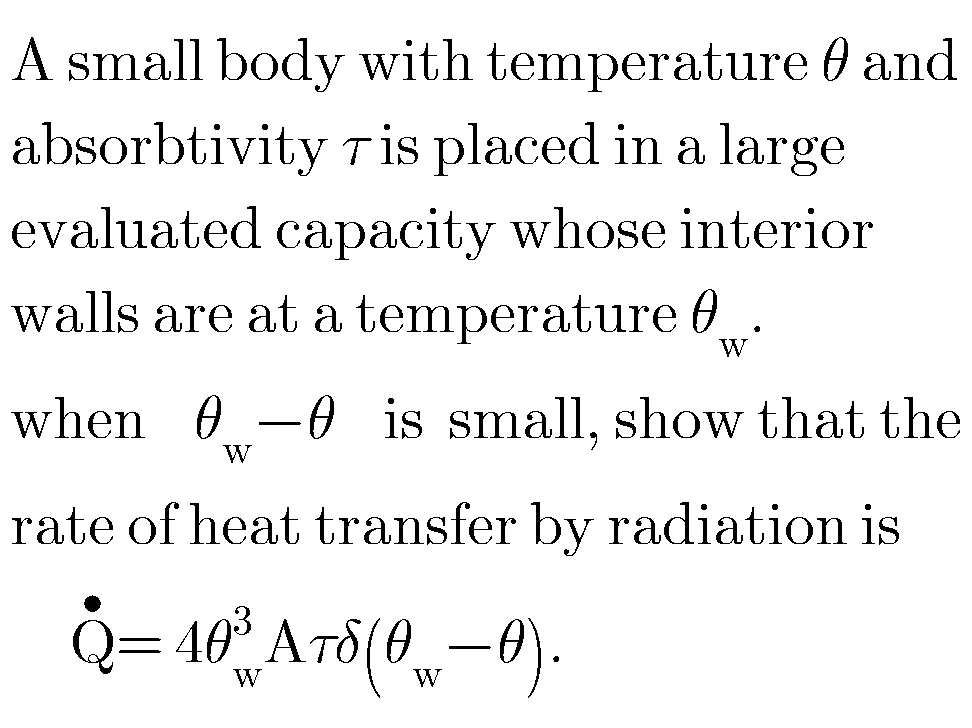
Question Number 58361 Answers: 0 Comments: 0
Question Number 58286 Answers: 0 Comments: 1
Question Number 58216 Answers: 1 Comments: 1
Question Number 58196 Answers: 1 Comments: 0
Question Number 54584 Answers: 2 Comments: 0

Question Number 52324 Answers: 1 Comments: 2
Question Number 51148 Answers: 1 Comments: 0
Question Number 51098 Answers: 1 Comments: 0
Question Number 50808 Answers: 2 Comments: 0

Question Number 49602 Answers: 0 Comments: 0
Question Number 47377 Answers: 1 Comments: 1

Question Number 47314 Answers: 1 Comments: 1
Question Number 46790 Answers: 0 Comments: 4

Question Number 45125 Answers: 1 Comments: 0
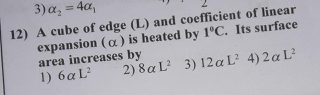
Question Number 45127 Answers: 1 Comments: 0

Question Number 45126 Answers: 0 Comments: 1

Question Number 44298 Answers: 1 Comments: 2
Question Number 42497 Answers: 0 Comments: 1
